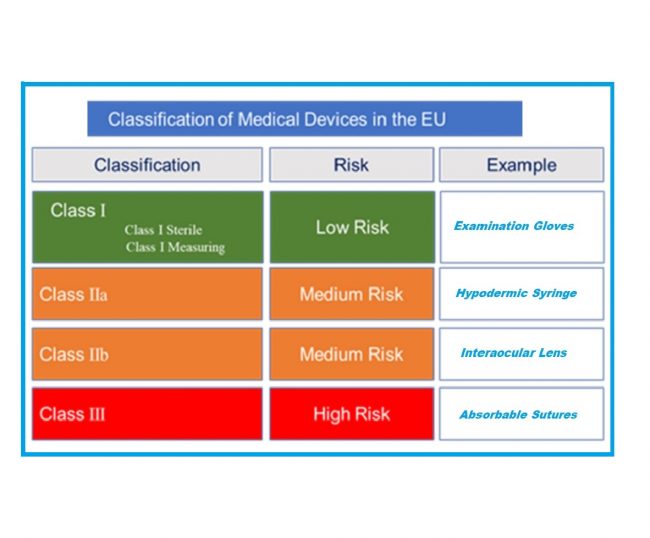
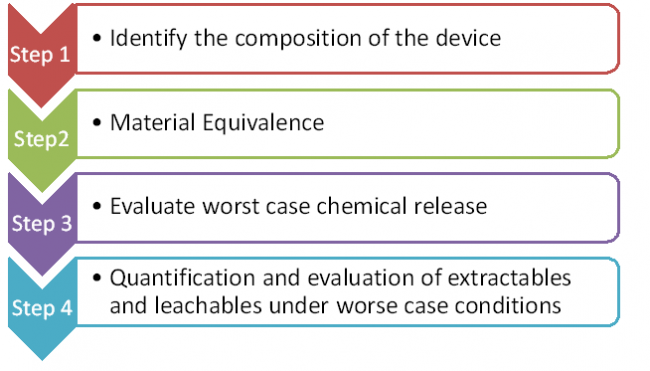
Medical Device Cyber Security Plan for Software
Medical Device Cyber Security Plan for Softwarebecome more competent in accessing, analyzing, communicating, and automation to improve the speed and quality of patient care. However, advancements with technological advances arise new risks and concerns. The risk of threats in both network and software is unavoidable through hackers. To assist manufacturers in developing adequate controls, the…