The EU Authorised Representative is mandatory for MDR & IVDR Medical Device CE Marking. EU Representative is applicable for all classes of medical devices. Medical Device Manufacturers that do not have a physical location in Europe must appoint a European authorized representative located within Europe.
EU Authorized Representative Name and Contact information must be visible on device information panels. Representative must be chosen based on their experienced and experienced handling of the regulations of Medical Devices and related regulatory requirements.
Article 11 section 1:
- Where a manufacturer is not established in a Member State, the device may only be placed on the EU market if the manufacturer designates a sole EU Authorised Representative.
Article 11 section 2 states:
- Shall be valid only when accepted in writing by EU AR.
- Shall be effective at least for all devices of the same generic device group.
- AR shall perform the tasks specified in the mandate agreed.
- AR shall provide a copy of the mandate to the Competent Authority upon request.
Article 11 section 3 :
- (a) Mandate shall require the EAR to Verify DoC and Technical File and confirm appropriate conformity assessment performed.
- b) Keep available a copy of Technical Documentation, DoC, and NB certificates at the disposal of the Competent Authority >10 / 10 years after the last placing on the market or >15 years for implantable devices.
- c) Register with SRN, within one week of any change maintain, re-confirm accuracy in the first year, and then every second year also check for correct UDI core data elements assigned / Entered Basic UDI-DI.
- d) Provide information/documentation to Competent Authority on request to demonstrate conformity in requested language.
- e) Forward to Manufacturer Competent Authority’s request for samples/access to a device and verify samples/access given.
- f) Cooperate with Competent Authorities in any preventive/corrective action to eliminate/mitigate risk.
- g) Immediately inform the Manufacturer about complaints and reports from healthcare professionals, patients, and users about suspected incidents.
- h) Terminate mandate if Manufacturer acts contrary to its obligations under this Regulation (inform Member State & Notified Body).
The main roles and Duties of EU Authorized representatives by Law are the following below:
- Authorization to place its name, address, and contact on device labels.
- Acting as your primary contact point for the EU Authorities and NB
- Register your devices with the Authorities before they are marketed.
- Make technical files readily available for the European Competent Authorities
- Notification of serious device incidents to the Competent Authorities
- Assistance with technical documentation or Clinical Evaluation Report
- Guidance on medical device Risk Analysis
- Device compliance with the MDR 2017/745 and IVDR 2017/746.
- Immediate notification of device incidents.
EUDAMED is known as European Databank for Medical Devices, which came into force in May 2011. EUDAMED is enforced for strengthening surveillance and transparency with regard to medical devices placed on the European market. EUDAMED acts as a central repository for information on market surveillance exchanged between the EU commission and national competent authorities.
As the EU Requirements are challenging, various governments worldwide require foreign manufacturers to provide a Certificate of Free Sale (CFS) or Free Sales Certificate (FSC) showing that their products are approved for sale in Europe. I3CGlobal organizes the Free Sale certificate from the EU Competent Authorities to all our existing customers. Free Sales Certificate can only be obtained on your behalf by your current EU Authorised Representative or UK Responsible Person.
In order to obtain a Free Sales Certificate, the following
- Mutually Signed EAR Agreement with us
- Technical File
- Complete test reports for Class 1 Devices
- EU Registration Document, If completed
- EUDAMED Registration & SRN Number
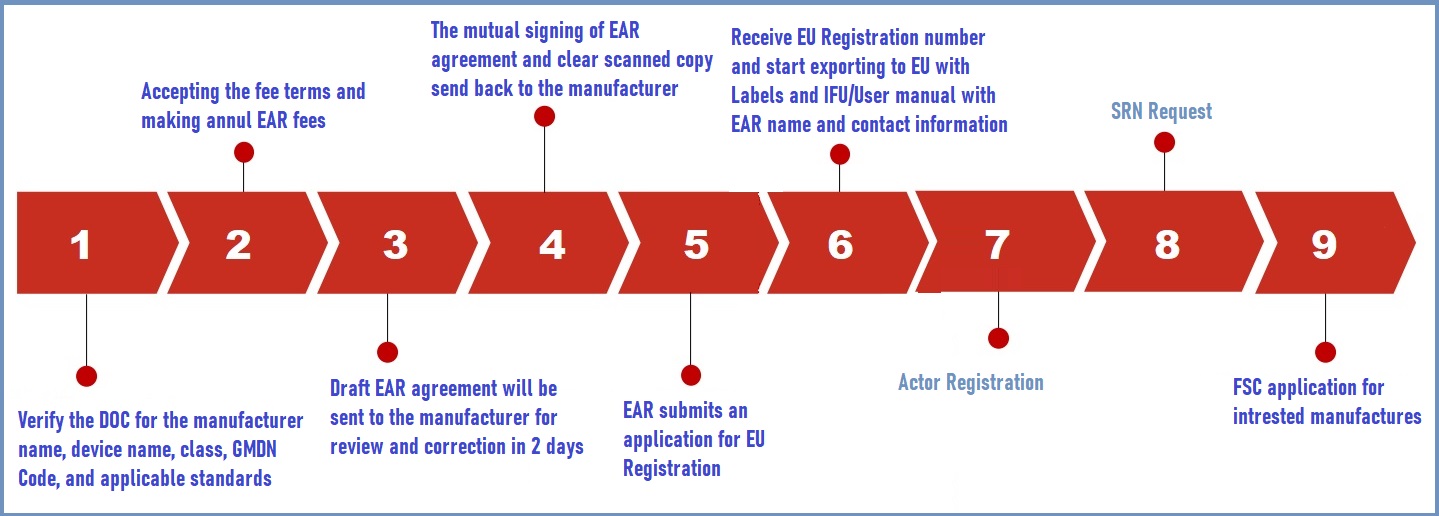
Yes, it is required at the port of entry. The European Authorized Representative has the authority to register non-EU manufacturers and medical devices with EU authorities. Will complete the EU Registration in the nation where the EC Rep is based. Only after the mutual signature of a EU Authorised Representative Service Agreement and submission of signed Declarations of Conformity (DOC) and Device Test Reports can EU Registration be filed for. The average time to register for the EU is two weeks.