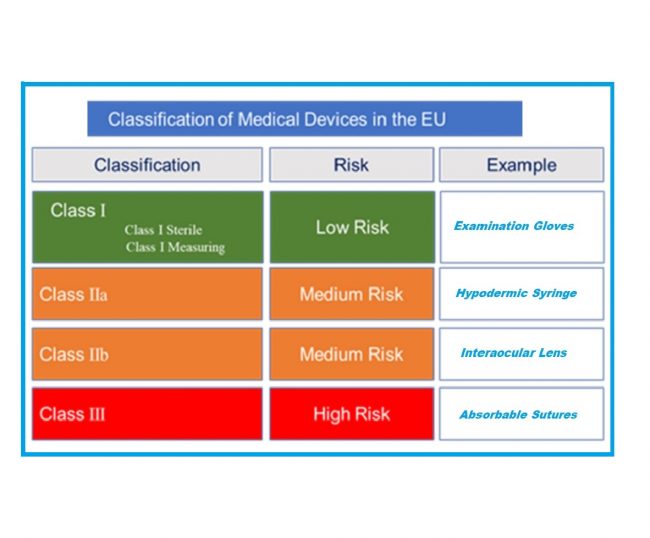
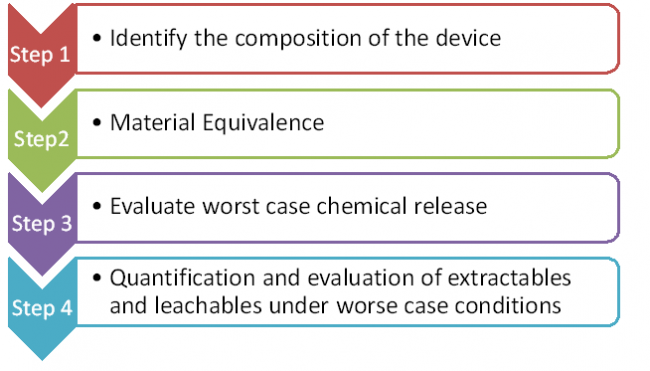
Medical Devices Certifications in UK
What is UKCA certification? The United Kingdom Conformity Assessed Certification, or UKCA Marking, is only legal in the United Kingdom. It was implemented in response to Brexit and went into effect on January 1, 2021. Manufacturers must adhere to the recommended UK product labeling in order for their items to be sold in Great Britain.…