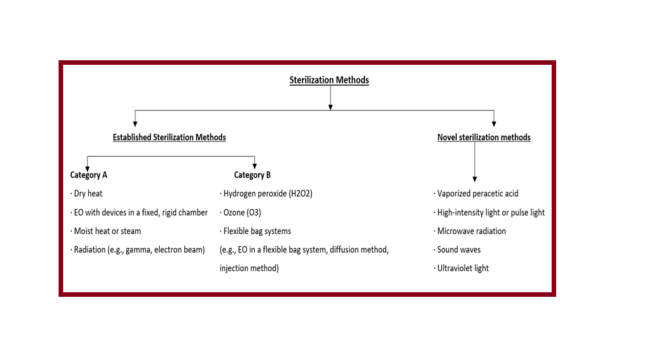
FDA Medical Device Sterilization
FDA Sterilization Guidance Medical Device Sterilization: To avoid disease transmission linked with the use of an item, sterilization destroys all microorganisms on its surface or in a fluid. Either a physical or chemical process that destroys or removes all microbial life in a specific area, including spores. Sterility: the reduction of anticipated levels of…